Extraction with supercritical CO2 as a solvent is an important separation technique used in the food, nutraceutical, cosmeceutical and pharmaceutical sectors: this process isolates certain active ingredients by subjecting the material to be extracted to high pressure in the presence of a gas, CO2, in the supercritical state.
The extraction with supercritical fluids is therefore a Green Technology that ensures environmental sustainability to the process and a high degree of quality and purity of the extracted product. It is a clean, selective technology; it does not require high temperatures and represents an alternative to common extraction with organic solvents, which are highly toxic to the environment and humans.
Carbon dioxide is used extensively in the chemical industry for its non-toxicity, renewability and stability, receiving considerable attention and investment for its potential as a solvent for supercritical state extractions (SC-CO2). Supercritical carbon dioxide is an alternative solvent for lipid extraction and has several uses: isolation of fats and oils from fish, oleaginous vegetable crops; extraction of nutraceutical and bioactive components from plants or vegetable waste; or to remove certain substances as in the case of caffeine from coffee, a process in which the extracted caffeine is subsequently used in soft drinks such as Coke. Thanks to its diffusivity in the biomass – close to that of a gas – supercritical CO2 works very well even with compounds of different polarity than oils, as in the extraction of polyphenols and anthocyanins. In these cases (e.g., polyphenols, caffeine, anthocyanins) CO2 in the supercritical state acts as a carrier, managing to solubilize the compound thanks to the combined action of CO2 in the supercritical state with a suitable polarity modifier such as water and / or ethanol.
The versatility of the phytochemical complex present in the plant world allows the development of countless food, cosmetic, pharmaceutical, functional food and nutraceutical products. The combined action of pressure and temperature, which modifies both the density and the degree of solubility of the supercritical fluid in the compound of interest, confers versatility to the SC-CO2. This allows the extraction of compounds sensitive to heat and oxidation such as polyunsaturated fatty acids (ω3, ω6), vitamins, cannabinoids, flavonoids, sterols, tocopherols and other compounds with high added value without damaging them. The extractive result is an extremely pure extract with very refined organoleptic characteristics.
Unlike conventional processes, the extraction is selective towards bioactive compounds and avoids impure end products and subsequent solvent recovery steps. Carbon dioxide is non-flammable and inexpensive, it is recycled, and its use on a large scale would stimulate the capture of atmospheric CO2 helping to balance greenhouse gas emissions.
CO2 in supercritical phase is selective towards apolar or weakly polar compounds, ideal for the extraction of biologically derived molecules such as those present in flowers or plant seeds. CO2 acts as a solvent when passing from the gaseous state at low density to a state called ‘supercritical’ with higher density: it reaches this condition at a temperature of 31.1°C and a pressure of 73.8 bar, losing its gaseous identity and assuming typical fluid properties, such as density and extraction capacity. After extraction, the pressure is lowered and the CO2 returns to a gaseous state, thus losing its solvent power and releasing the solute substances, now available in a pure and concentrated form. A strict control of pressure and temperature of the process allows management of CO2 density and therefore the extraction capacity, being able to select certain compounds from others.
The use of supercritical extraction technologies produces extremely pure products that guarantee the high-quality standards required in the pharmaceutical, nutraceutical and food sectors, as well as guaranteeing environmental sustainability of the entire production process, from plant to extract.
—————————————————————————————-
Notes
SCF (supercriticalfluid) is defined as a low molecular weight fluid having a critical temperature close to ambient (TC ~ 10 ÷ 40 °C) and a critical pressure not too high (PC ~ 40 ÷ 60 bar). Light hydrocarbons have these physical properties but are flammable and toxic. Certain chlorofluorocarbons lend themselves to the purpose, but are relatively expensive when obtained at high purity and are toxic in terms of environmental acceptability.
Carbon dioxide, while having a slightly higher critical pressure (PC = 72.1 bar), offers other advantageous properties that make it the most widely used fluid in SC applications:
- Non-flammability;
- Environmental acceptability;
- Non-toxicity;
- Low cost even at high purities.
The possibility of modulating the density of SCFs thanks to modest variations in temperature and pressure, especially around the critical point (PC), represents the characterizing factor of these fluids. This introduces pressure as an additional variable in the control of a separation and / or extraction process.
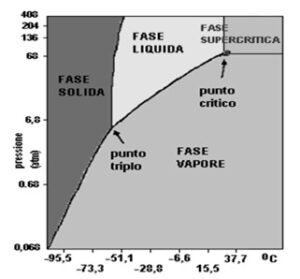
From within the supercritical state, possible combinations of pressure and temperature vary the solubilizing properties of carbon dioxide.
Historical background:
In 1822 Baron Charles Cagniard de la Tour discovered the critical point of a substance in his experiments with cannons. Listening to the discontinuities in the noise of a flint sphere in a sealed cannon containing fluids at various temperatures he observed the critical temperature. Above this temperature the densities of the liquid and gas phases become equal and the phases themselves become indistinguishable, resulting in a single supercritical fluid phase. The phases of an element or compound can be studied on special phase diagrams such as the one on the left.
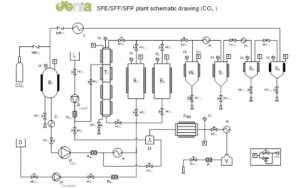
Figure 1: R&D plant configuration.
Legend: storage tank (B1); tower (T1); extractor (Ex); liquid tank (L); safety unit (k); CO2 pump (PCO2); co-solvent pump (Pcosolvent); liquid pump (Pliquid); condenser (C1); Cooler (C2); separator (Sx); heat exchanger (Hx) ;valves (HVx); membrane valves (MVx); non-return valves (NVRx); Coriolis flow meter (FLx); gas flow meter (Fx); co-solvent tank (D); pasteurization reactor (Pas); Mixer (M).
Ultimately, a fluid in the supercritical state is characterized by chemical and physical properties intermediate between those of a liquid and those of a gas. In particular, the fluid has solvent properties similar to those of a liquid and transport properties common to those of a gas.
At present it is our belief that the use of supercritical fluids has interesting industrial prospects in cases where:
- one intends to operate economies of scale to reduce the unit industrial costs of the final product, (typical situation for several edible oils);
- there is a high added value of the compounds to be treated;
- there are thermolabile substances that undergo alteration with traditional extraction processes, resulting in loss of activity in the product;
- one wants to obtain high extraction yields;
- one intends to carry out several extractions of different classes of compounds from the same matrix.
The use of supercritical fluids (SCFs) as solvents in separation techniques dates back to the last two decades and has found wide application in extraction operations (SFE-Supercritical Fluid Extraction).
The limitation represented by the high cost of plant has always conditioned the use in the commercial field and only recently, the increase in raw materials, labor, and energy resources has made possible their exploitation.
After the first industrial applications of supercritical fluids (decaffeination, purification …) and two decades of research and applications in the food industry, SCFs are now also used in the pharmaceutical industry.
The use of SCFs in industrial processes, however, remains closely linked to issues of purification, extraction, fractionation, solubilization, and support to other technologies.
The fundamental properties of extraction with supercritical fluids can be summarized as follows:
- solvent power and selectivity of the fluid employed;
- the employed fluid is a non-flammable gas with full environmental acceptability;
- the operating temperatures make it a “cold” technology;
- it is possible to modulate the pressure as well as the temperature in order to optimize both the extraction and separation processes.
In addition, with reference to the production of flavors, essences and oils, a quick analysis allows to assert that, compared to traditional production systems, SFE completely overcomes the issues related to the use of organic solvents and steam extraction.
In the first case, a part of the solvent remains solubilized in the finished product; in the second case, the high temperatures and the reactivity of the water are such as to generate changes in the composition of the product, both quantitatively and qualitatively.
The plants used for the conduction of the extraction process with supercritical fluids are composed of equipment (volumetric pumps), autoclaves (extractors, separators, storage tanks), fittings and pipes suitable to operate at high pressures (up to 600 bar) and temperatures (-10 + 90°C).
The main units of a typical plant, shown in the process diagram in Figure 1, are highlighted as follows:
- storage tank, adequately refrigerated, for the recovery of the liquid solvent (B1);
- volumetric pumps to bring CO2, co-solvent or liquid charge (PCO2, Pcosolvent;Pliquid) to the desired pressure;
- extraction autoclaves (E1 25 L, E2 6 L) where the physical extraction process takes place;
- separation autoclaves (S1, S2, S3) used for extract fractionation;
- fractionation tower (T1) for the separation of liquid charges with recirculation;
- heat exchangers (Hx); condenser (C1) and cooler (C2)
- thermal services, both hot (boiler) and cold (refrigeration unit)
- Coriolis effect mass flow meters (FLx)
The entire extraction process by supercritical carbon dioxide can be summarized as follows:
the liquid carbon dioxide contained in the tank B1 (Figure 1) is pressurized by the pump PCO2, evaporated by the exchanger H1 and delivered to the extraction reactor Ex, which contains the raw material to be extracted and where the actual extraction takes place.
The solvent, more or less saturated with extract, is laminated through the lamination valve MV1, through which the carbon dioxide loses its solvent power resulting in precipitation of the solute in the separation vessel S1.
A second and a third stage of cyclonic separation S2 and S3 allows to collect the lighter fractions of the extract not precipitated in S1. The solvent leaving S3 is recycled in B1. An appropriate choice of T, P and Q conditions allows a very selective solubilization of the molecules contained in the raw material loaded in Ex.
The density of supercritical CO2 at 40°C ranges between 480Kg/m3 at 90 bar, 700 kg/m3 at 120 bar, 840 kg/m3 at 240 bar, 930 kg/m3 at 350 bar up to 1.020 kg/m3 at 600 bar.